As beautification and protection of substrate coating itself is in need of protection, in particular for hazardous substances in the environment such as heat energy, oxygen, water, and light. Although most of the polymers do not absorb ultraviolet light directly, but all the paint more or less contains materials that absorb UV light, causing resin peroxide substance.Ultraviolet radiation (which comes from the Sun) will make the coating’s performance and to make it worse. Artificial light will produce the same effect. Ultraviolet rays harmful to the damage the polymer’s chemical bonds. This makes resin degradation leading to changes such as loss of gloss paint, powder, discoloration, yellowing, loss, loss of physical performance and protection. This chemical process called photo-oxidation. UV-LED light oxidation process Photo-oxidation are two important processes. First is the photodissociation process, the complex processes that exist in multiple steps, including UV, and then open the molecules key to the formation of free radicals. The second process is the oxidation process, formed in the photolysis of radicals react with oxygen to produce superoxide free radicals.In the photo-oxidation process has 5 steps. In the following description, the R stands for paint resins easily oxybenzone in parts.
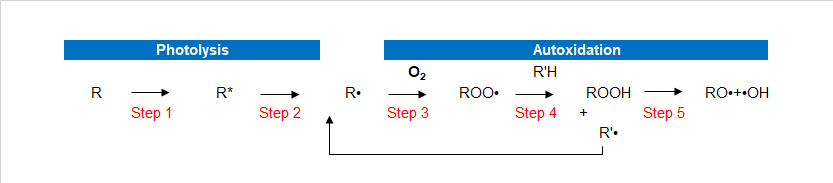
- Step 1
- Step 2
- Step 3
- Step 4
- Step 5
Paint absorbs UV radiation. UV-absorbing substances (such as resin or impurities) will be absorbed into high energy excitation energy of the excited state (R*). These excited molecules are very active, and goes through a variety of processes. Process or make two of the same excited state back to normal, or key is split.
If the molecule cannot become the norm, the key cracking formation of free radicals (R ·).
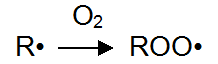
Formed in the photolysis of radicals react easily, such as oxygen to produce superoxide free radicals. This process is called oxidation.
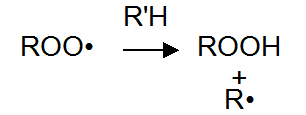
Peroxy radicals by hydrogen bonds pull attacks the polymer backbone (R’H), formation of peroxides and free radicals. These free radicals can react with the oxygen in the third generate additional oxidative free radicals.
Peroxy radicals by hydrogen bonds pull attacks the polymer backbone (R’H), formation of peroxides and free radicals. These free radicals can react with the oxygen in the third generate additional oxidative free radicals.
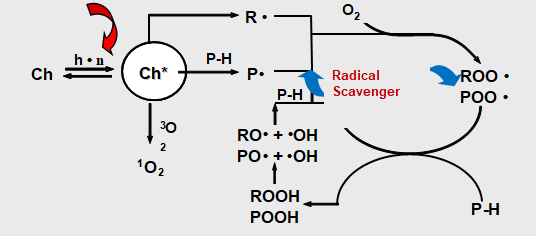
Types of light stabilizers
There are two types of light stabilizers. One is UV absorber which is to absorb harmful UV light to protect the coating. The other is hindered amine light stabilizer which is to capture free radicals to avoid coating degradation.
- UV absorbers is to absorb UV light in competition with the chromophores which are part of the polymer backbone to prevent degradation. They are colorless or almost colorless additives, which have a strong absorbability in the ultraviolet part of the spectrum. UV absorbers can dissipate light energy as thermal energy.
- Hindered Amine Light Stabilizers (HALS) is to capture free radicals before subsequent reactions leading to degradation can take place. HALS can impede thermo-oxidation. The polymer contains the HALS will still keep the resistance to photo-degradation even run of the HALS. The explanation for this phenomenon is that HALS’ oxidation products, such as hydroxyl-amine and aminoether, can inhibit photo-degradation. Hydroxyl-amine and aminoether are all able to capture peroxide free radicals.
Share this page
