[:en]By the preparation of colored paint, a good dispersion quality is one of the most difficult factors. The dispersion process consists of converting dry pigments into pigment dispersion, which must be fine and sufficiently stable to achieve the final coloristic properties and stability. This is a complex process there resin, type of pigment, solvents and the use of dispersing agents are playing here an important role.
1. Dispersion process
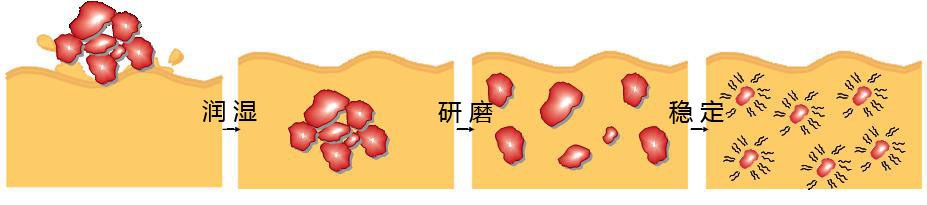
The dispersion process of a pigment in liquid coatings can be divided into the three processes:
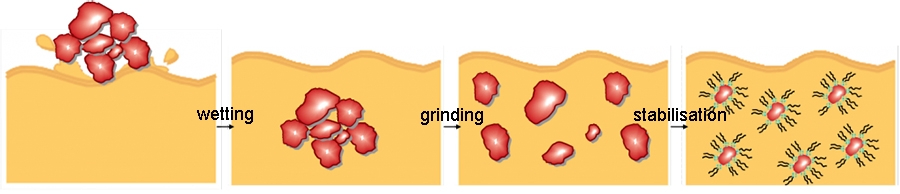
Wetting
The air and moisture covering the pigment is replaced by the resin solution. The solid/gas interface (pigment/air) is transformed into a solid/liquid interface (pigment/resin solution).
Grinding stage
By high shear forces the pigment agglomerates are broken up into smaller units, preferable primary particles.
Stabilization
The pigment dispersion is stabilized by dispersing agents in order to prevent the formation of uncontrolled flocculates. The resultant suspension is stabilized due to the adsorption of binder species or molecules at the pigment surface.
Pigment wetting: The air and moisture covering the pigment is replaced by the resin solution. The solid/gas interface (pigment/air) is transformed into a solid/liquid interface (pigment/resin solution).
Grinding stage: By high shear forces the pigment agglomerates are broken up into smaller units, preferable primary particles.
Stabilization: The pigment dispersion is stabilized by dispersing agents in order to prevent the formation of uncontrolled flocculates. The resultant suspension is stabilized due to the adsorption of binder species or molecules at the pigment surface.
Dispersing additives, which adsorb on the pigment surface, facilitate liquid/solid interfacial interactions and help to replace the air/solid interface by a liquid medium/solid interface.
The grinding process can be regarded as a de-flocculation process. In the absence of stabilizing agents, effects such as reduced color strength, decreased gloss, and altered rheology may occur.
1.1 Stabilization of pigment dispersions
The pigment dispersion what is achieved in the last step will be used later in the let down system where it should stay stable during storage and later during the application and film formation.
There are two principal mechanisms for the stabilization of pigmented dispersions described:
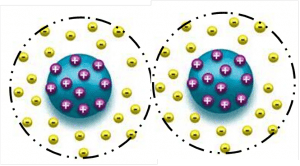
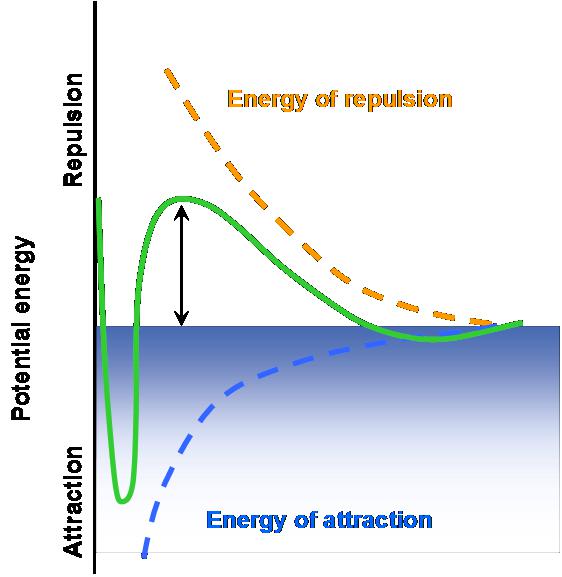
1.1.1 Electrostatic stabilization is only working in a water based application. When two particles having the same charges approaching each other will result in a repelling effect. The resulting Coulomb-repulsion of the charged particles allows the system to remain stable.
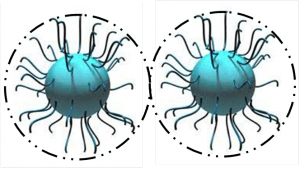
1.1.2 Steric stabilization suited for water and solvent based systems is when pigments are sterically stabilized (the surface of the solid particles are completely covered by polymers) making particle-to-particle contact impossible. Strong interactions between polymers and solvents (organic solvent or water) prevent the polymers from coming too closely into contact with one another (flocculation).
Steric stabilization relies on the adsorption of a layer of resin or polymer chains on the surface of the pigment.
One fundamental requirement of steric stabilization is that the chains are fully solvated by the medium. This is important because it means the chains will be free to extend into the medium. In systems where the chains are not so well solvated they will prefer to lie next to each other on the surface of the pigment, providing a very much smaller barrier to inter-particulate attraction what will result in much easier flocculation
2. Dispersants Families
The choice of the dispersing agents for the pigment stabilization is a key issue in the coating and ink industry. Formulators have to find the most suitable products for their formulation taking into account the final application of their coating, the coating system (water based, solvent based, etc.) and the other additives.
The role of the dispersing agents is to enhance the dispersion process and ensure a fine particle size in order to stabilize pigments in the resin solution. As explained earlier, an efficient dispersant has to perform the three main functions: pigment wetting, dispersing, and stabilizing. Dispersing agents generally differ for aqueous and solvent-based coatings.
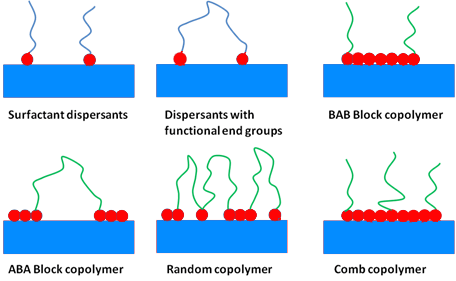
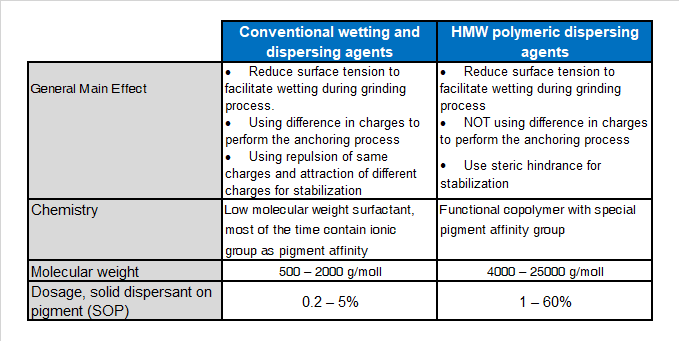
In term of chemical structure one can divide dispersing agents into the two following classes:
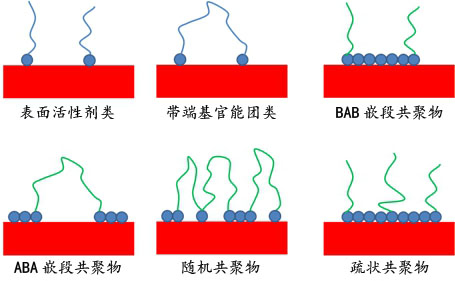
- Surfactants, also called low molecular weight dispersing agents
- Polymeric dispersants, also called high molecular weight dispersants
The main differences of those two types of dispersants being the molecular weight, the stabilization mechanism and the resulting let down stability.
In addition polymeric dispersing agents have multiple anchor groups where surfactant like dispersing agents more related to a polar head with a side chain for the compatibility.
2.1 Polymeric Dispersants
Polymeric dispersants stabilize paints, coatings and ink systems via a steric stabilization mechanism.
They must have specific anchor groups capable of being strongly adsorbed into the particle surface and must contain polymeric chains that give steric stabilization in the required solvent or resin solution system.
Polymeric dispersants differentiate themselves from the other types of dispersing agents through considerably higher molecular weights. Because of its structural features, a polymeric dispersant is bound to numerous sites at the same time, forming durable adsorption layers upon many pigment particles. Optimal steric stabilization is achieved when the polymer chains are well solvated and properly stretched, therefore they must be highly compatible with the surrounding resin solution. If this compatibility is obstructed, the polymer chains collapse causing the steric hindrance and the resulting stabilization to be lost.
In order for additives to be effective, the adsorption into the pigment surface must be durable and permanent. The surface properties of the pigment particles are therefore crucial to the additive’s effectiveness:
With pigments possessing high surface polarities, such as inorganic pigments that are ionically constructed, the adsorption of any dispersing additive is relatively easy.
However, for pigments with nonpolar surfaces, such as organic pigments whose crystals are composed of nonpolar individual molecules, a proper adsorption is rather difficult to obtain with conventional additives. The wide range of anchor groups that polymeric dispersants provide make them very efficient to anchor on pigments with nonpolar surfaces.
In the traditional method of stabilizing pigments in water, the stabilizing charges used are often disturbed by impurities, such as other ions, or the presence of other pigments with different zeta-potentials. This leads to a destabilizing effect, caused by the reduction of the repulsive forces. Steric stabilization can avoid this issue, making polymeric dispersants very useful for dispersing all types of pigments, even the organic ones, that are very difficult to be deflocculated by traditional wetting and dispersing additives.
The nature of the polymeric chain is critical to the performance of polymeric dispersants. If the chains are not sufficiently solvated, then they will collapse on to the pigment surface allowing the particles to aggregate or flocculate. The need for compatibility with the medium extends throughout the final drying stages of any applied coating. If it ceases to be compatible, flocculation may occur leading to a decrease of surface properties such as losses in gloss and tinctorial strength, etc.
The molecular weight of the polymeric dispersant must be sufficient to provide polymer chains of optimum length to overcome Van de Waals forces of attraction between pigment particles
Finally, for good surface coating properties and performances, the polymer must be fully compatible with the coating resin after the solvent has evaporated off and the resin has been cross-linked.
2.2 Low molecular weight dispersants (surfactants)
Surfactant dispersants are conventional low molecular weight dispersing agents. Surfactant molecules are able to modify the properties and, in particular, they lower the interfacial tension between the pigment and the resin solution.
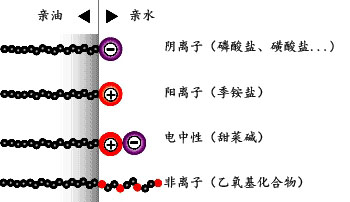
This surface activity arises because the surfactants’ structure consists of two groups of contrasting solubility or polarity. In aqueous systems, the polar group is known as a hydrophilic group and the non-polar group as hydrophobic or lipophilic. In non-aqueous systems, the polar group is known as the oleophobic group and the non-polar group as oleophilic. Surfactants are classified according to their chemical structure and, more specifically, their polar group: anionic, cationic, electroneutral and non-ionic.
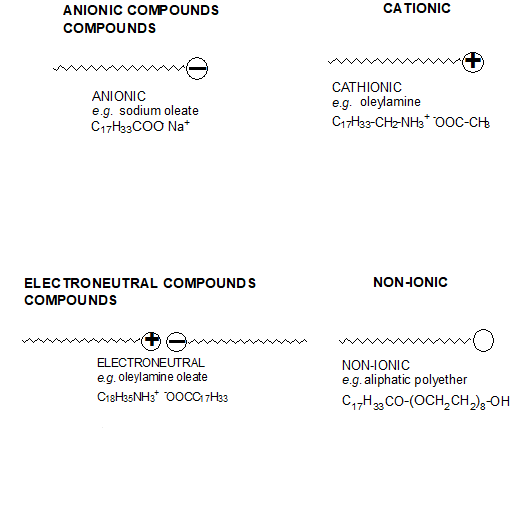
As with the polymeric dispersing agents, their effectiveness is determined by:
- The absorption of the polar group onto the pigment surface. The anchoring groups can be amino, carboxylic, sulfonic, phosphoric acids or their salts.
- The behaviour of the nonpolar chain in the medium surrounding the particle. This part of the molecule (aliphatic or aliphatic-aromatic segments) must be highly compatible with the binder system.
The stabilisation mechanism of surfactant-like dispersing agents is electostatic: the polar groups forming an electrical double layer around the pigments particles. Due to the Brownian movement the pigment particles frequently encounter each other in the liquid medium thus having a strong tendency to re-flocculate on the let down stage.
Because of their chemical structure (eg: low molecular weigth) and the electrostatic method of stabilization, surfactants may cause the following defects:
- Water sensitivity: Surfactants generally have a tendency to provide water sensitivity to the final coating, thus making them inappropriate for use in outdoor applications.
- Foam formation: Many surfactants generate foams which lead to surface defects (eg. fish eyes, craters) on the final coating. If foaming occurs at the milling stage it can also cause a loss of production capacity.
- Interference with intercoat adhesion.
Over the past years specific surfactants have been developed to minimize these defects, and
some provide other advantages to the final paints such as defoaming/dearation or difficult substrate wetting.
The most widely used surfactants for pigment dispersion in coating formulations are:
- Fatty acid derivatives
- Phosphate esters
- Sodium polyacrylates / polyacrylic acid
- Acetylene diols
- Soya lecithin
Main difference between LMW dispersant and HMW dispersants are:
3 Required amount of dispersant
Dispersing agents are not just additives to conventional millbases. The choice of the most suitable dispersing agents is sometimes difficult and their usage require sometimes specific guidelines.
The choice of dispersant is also related to the surface nature of the pigment. The polarity of the surface of the pigment differs from organic (non-polar) to inorganic (more polar), and this means that the nature of the dispersant anchor group is critical for optimum absorbtion. The choice of anionic anchor group should allow for better performance with inorganic pigments and a cationic anchor group should be more appropriate for organic pigments.
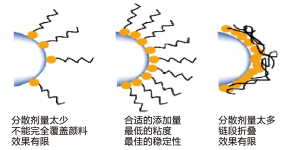
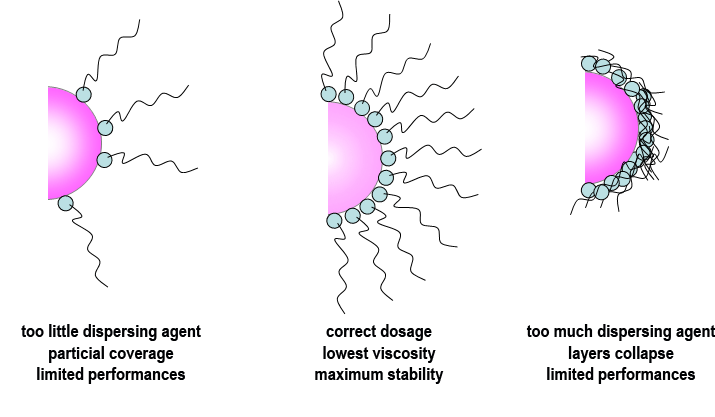
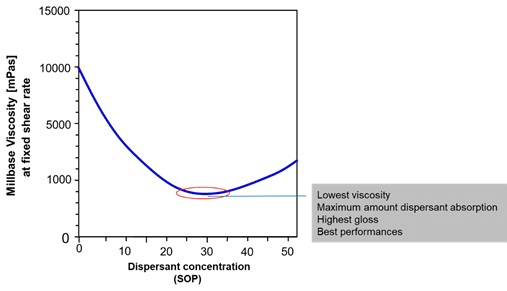
The surface area of the pigment also affects the level of dispersant used, and in general, if too little is used then the full benefits will not be realised. If too much is used, it can be shown that the thickness of the protective barrier is actually reduced as a result of overcrowding on the pigment surface. Therefore the use of an excess level of dispersant actually leads to final coating properties which are inferior to those obtained with an optimised dosage.
As a general rule, 2-2.5mg of polymeric dispersant, per square meter of pigment surface area will be close to the optimum amount required.
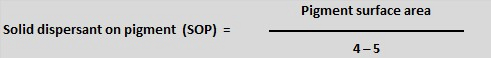
A ladder series of polymeric dosage levels should be evaluated based around this 2-2.5mg/m2 level. Measurement of dispersion viscosity will show a minimum at the optimum dosage; although it is also possible to measure gloss or colour strength of the coating which will show a maximum at the same optimum dosage.
[:zh]1.分散技术
有色油漆的生产时,好的分散质量是其中一个最困难的因素。 分散过程包括将干燥颜料转换成颜料分散体,而颜料分散体必须是分散完全的和足够稳定的,以实现最终的色彩性能和稳定性。 这是一个复杂过程,其中有树脂、颜料、溶剂,分散剂的使用在这扮演一个重要角色。
2.分散过程
带有高饱和度和着色力的高质量涂料的特点是良好的颜料分散,最优化颜料粒径和长期稳定性。
在液体涂料中,颜料的分散过程可分为三个过程:
颜料润湿: 覆盖颜料的空气和湿气被树脂溶液所取代。 固/气界面(颜料或空气)被转换成一个固/液界面(颜料或树脂溶液)。
研磨阶段:通过高剪切力的颜料结块被分解成更小的单位,最好是初级粒子。
稳定:通过分散剂稳定的颜料分散体,以防止形成不受控制的絮凝。通过使树脂吸附在颜料表面而形成悬浮稳定。
2.1润湿阶段
湿润步骤包括用树脂溶液取代覆盖颜料的空气和湿气。
通过颜料粒子与树脂的互动来润湿颜料的原始颗粒从而增强液体涂料的性能。分散剂,通过吸附在颜料表面,帮助液/固表面的互动并且取代气/固表面。
润湿的效率主要取决于颜料和介质的相对表面张力及其粘度。吸附的机理主要取决于颜料的化学特性和分散剂的种类。
2.2 研磨阶段
润湿阶段之后,就必须去聚集和去颜料颗粒结块。这通常是通过高剪切研磨设备提供机械作用。
在研磨阶段,必须克服内部凝聚力的结块。通过剪切使系统产生更小的粒子,通过如研磨机等的剪切力作用来破坏颜料的附聚体。
颜料通过机械剪切被分解成独立的微粒,导致与载体接触的表面积更高,因此需要较大数量的添加剂来湿润其表面. 一旦分散,初级粒子有一种倾向,重新凝聚。这个过程被称为絮凝。
在研磨过程可以被看作是去絮凝过程。缺乏稳定会导致如着色力降低,光泽度下降,并可能改变流变性。
2.3颜料分散体的稳定
颜料分散体在配漆后的储存阶段或在应用和涂膜形成时,都应该保持稳定。
稳定是通过吸附在颜料表面的稳定分子,使粒子具有排斥力足以阻止其他粒子太接近接引起凝聚。
两种稳定颜料分散的主要机制描述:
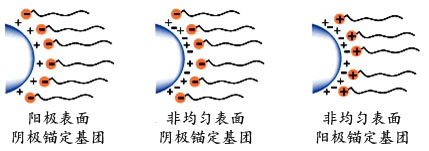
1. 静电稳定只在水性体系作用。 当有二个相同电荷的微粒接近时会导致一个排斥的作用。 由此产生的库伦相斥力的带电粒子使系统保持稳定。
图2 电荷相斥
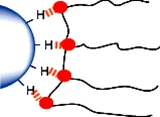
2.一个更强大的能用于水性和溶剂型系统系统的是颜料空间位阻稳定(固体颗粒的表面完全被聚合物覆盖)使粒子对粒子的接触变不可能。聚合物和溶剂之间的强相互作用(有机溶液或水)防止聚合物互相紧密的接触(絮凝)。
图3 空间位阻
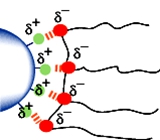
图4 稳定机制
空间位阻稳定取决于吸附在颜料表面的树脂层或聚合物段。其根本要求是,聚合物链段被充分溶解。这是重要的,因为这意味着聚合物链段将是自由延伸到其中。在聚合物链段溶解不好的系统中,聚合物链段倾向在颜料的表面相互紧挨着,其排斥力不足以克服颗粒间的吸引力,会导致絮凝。
3. 分散剂家族
在涂料和油墨行业,分散剂的选择是一个关键问题。设计者要综合考虑其涂料的最终应用,涂料的体系(水性,溶剂型)和其他添加剂的关系来找到最合适的产品。
分散剂的作用是增强分散过程,确保细颗粒大小,以稳定在树脂溶液中的颜料。如前所述,一个有效的分散剂起三个主要功能:颜料润湿,分散,稳定。分散剂一般分为水性涂料用和溶剂型涂料用。
根据化学结构可以分为分散剂为以下两个类型:高分子分散剂和表面活性剂。这两类分散剂主要区别在于分子量,稳定的机制和存储的稳定性。
3.1高分子分散剂
高分子分散剂是以空间位阻稳定机制来稳定油漆,涂料和油墨系统。
他们必须有特殊的锚定基团能够强烈地吸附到颗粒表面,并且必须包含能为所需的溶剂或树脂溶液体提供空间位阻稳定的高分子链段。
图5 分散剂的种类
高分子分散剂以相当高的分子量来区分自己与其他类型的分散剂。
由于其结构特点,高分子分散剂必将同时吸附在颜料表面的多个点,形成持久的吸附层。理想的空间位阻稳定是以高分子链的良好被溶解和适当地伸展来实现,因此他们必须与周围树脂溶液高度兼容。如果这兼容性被妨碍,造成高分子链的空间位阻崩溃以及使得稳定丧失。为了使添加剂更有效, 要求其吸附颜料表面的能力必须是持久和永久的。因此颜料粒子的表面性质关系到添加剂的功效。具有较高的表面极性的颜料,如无机颜料是离子键结构,是比较容易吸附各种分散剂。
然而,对于非极性表面的颜料,如有机颜料的晶体是由非极性的个别分子组成,是很难与传统的添加剂获得一个适当的吸附。宽广范围的锚定基团之高分子分散剂对非极性表面的颜料提供非常有效的锚定。
在水中稳定颜料的传统方法,稳定电荷往往被杂质所干扰,如等离子,或存在其他不同贝塔电位的颜料。这导致了破坏性影响,造成排斥力的减少。空间位阻稳定可避免此问题。使高分子分散剂非常适用于所有类型颜料的分散,包括有机颜料,这些用传统的润湿剂和分散剂是很难防止絮凝的。
3.1.1 空间位阻链段
聚合物链段的特性对聚合物分散剂的性能至关重要。如果其链段不能完全溶解,则在颜料表面崩溃从而引起颗粒聚集或絮凝,导致失光,着色力降低等。
聚合物分散剂的分子量要能提供足够长的链段来克服范得华力。
如果链段太短,则不能提供足够厚的壁垒来防止絮凝。这就意为着太小的分子量会导致分散体的不稳定并会导致粘度上升和着色力降低。
如果链段太长,则它们会有折反的趋势。这意为着太大的分子量会导致性能的降低。理想的链段应该可以在体系中自由移动。如前所述,一端带有锚定基团的链段显示最佳的空间位阻。
最后,为了取得更好的涂料性能,聚合物分散剂必须与涂料树脂体系完全相溶,即便在溶剂挥发和树脂交联阶段。
3.1.2 锚定基团
由于颜料表面的性质不同,根据其化学类型,许多不同的化学基团可作为高分子分散剂的锚定基团。高分子分散剂的锚定基团广泛的可能性,使得它对无机颜料和极性颜料分散好。实际锚定作用可以透过多种机制。
(1)锚定作用通过离子或酸性/碱性基团
当颜料颗粒具有相对活泼的表面(如:无机颜料),它有可能形成离子对键,一边是颜料粒子表面带电位, 另一边是分散剂上带反电荷的原子或官能团。它的情况显示在图6a而且是有效的,因为有机溶剂通常具有较低的介电常数,不利于电荷分离。
图6 离子或酸性/碱性基团机制
事实上,许多无机颜料粒子表面相当地不均匀,既有正电荷和负电荷的部分。因此普遍地发现,可以使用带正电荷或负电荷的锚定基团的高分子分散剂能更好的分散颜料。如图6b和6c所示。
可被用来锚定聚合物链于带电的或酸性/碱性表面的例子包括胺;铵和季铵基团;
羧酸,磺酸,磷酸,酸性基团及其盐类,以及硫酸和磷酸酯基团。
(2) 錨定作用通过氢键基团
虽然大多数有机颜料和一些相对惰性的无机颜料(如石英)在其表面没有电荷,但他们可能有氢键供体或受体基团,如酯类,酮类和醚类。因此在颜料和聚合物分散剂的锚定基团间可能形成氢键。即使个别氢键力较弱。但如果聚合物分散剂含有许多氢键供体和受体,还是会在颜料粒子和分散剂间产生一个强的相互作用。
图7 氢键作用机制
多胺和多元醇均可被用来通过氢键作用来锚定颜料,其可能是氢键供体或受体。聚醚可被用来通过氢键接受体锚定颜料。
(3) 锚定作用通过极性基团
有机颜料表面的极性基团与聚合物分散剂锚定端的极性基团之间会产生相互作用。同样,这些相互作用往往是相对较弱,但一个高分子分散剂拥有多个这样的基团会强烈的相互作用。
聚氨酯是常用的极性锚定基团
图8 机性基团机制
3.2表面活性剂分散剂
表面活性剂是传统的低分子量分散剂。表面活性剂分子可降低颜料和树脂溶液之间的表面张力。
这种表面活性的产生是因为表面活性剂结构包括两组不同的可溶性或极性基团。
在水性体系中,极性基团被称为亲水基,非极性基团被称为如疏水基或亲油基。在非水系统中,极性基团被称为疏油性和非极性基团被称为的亲油性。表面活性剂按其化学结构,更具体地说,按极性基团分为:阴离子,阳离子,电中性和非离子型(见图1)。
图9 按机性基团分类表面活性剂
同高分子分散剂,其有效性取决于:
●颜料表面吸附的极性基团,锚定基团可以是氨基,羧基,磺酸,磷酸或其盐类。
●在粒子周围介质中的非极性链的表现。这部分的分子(脂肪族或脂肪族 – 芳香族段)必须与树脂体系高度兼容。
表面活性剂类分散剂的稳定机制是电荷位阻:极性基团形成的双电层围绕颜料粒子。由于布朗运动,在液体介质中颜料粒子经常相互碰撞,因而在调漆阶段有再絮凝的强烈倾向。
由于其化学结构(如:低分子量)和电荷位阻的稳定,表面活性剂可能会导致以下缺陷
●水敏感性:表面活性剂一般对涂层有水敏感性的倾向,使它们不适用于在户外应用。
●泡沫的形成:在涂层中许多表面活性剂会产生泡沫而导致的表面缺陷(如鱼眼,缩孔)。如果泡沫出现在研磨阶段,也会造成生产能力的损失。
●影响层间附着力
在过去几年表面活化剂已发展至尽量减少这些缺陷,并提供其他一些优势给最后的油漆如消泡/脱泡或是困难的基材湿润。
4.分散剂的用量
选择最合适的分散剂通常是很困难的,它们的用量也需要特别的指导。
分散剂的选择也与颜料的表面特性有关。颜料表面的极性也会随有机颜料(非极性)到无机颜料(极性)而差异,这意为着分散剂的锚定基团的选择对于最佳的吸附也很重要。选择阴离子的锚定基团对无机颜料比较好,而选择阳离子的锚定基团对有机颜料更合适。
颜料的表面积也决定分散剂的用量。总的来说,如果用量太少,则达不到最佳效果。如果用量太多,则空间位阻的厚度会由于过分拥挤而降低,反而达不到最佳效果。
图10 分散剂的添加量
通常,最佳的合适用量范围:每平方米的颜料表面积使用2-2.5mg的聚合物分散剂。
应根据2-2.5mg/m2聚合物用量水平附近作梯状评估。色浆粘度的测量可以为选择最佳量的最低值提供依据,虽然它也可以通过测量光泽或色彩强度,来显示一个最佳剂量的最大值。
比如:酞菁蓝颜料的表面积为 50 m2/g
那么,一个典型的剂量是:
•酞菁蓝颜料30.0(即:10%活性分散剂对颜料重量)
•高分子分散剂:3.0
[:de]By the preparation of colored paint, a good dispersion quality is one of the most difficult factors. The dispersion process consists of converting dry pigments into pigment dispersion, which must be fine and sufficiently stable to achieve the final coloristic properties and stability. This is a complex process there resin, type of pigment, solvents and the use of dispersing agents are playing here an important role.
Dispersion process
The dispersion process of a pigment in liquid coatings can be divided into the three processes:
Pigment wetting: The air and moisture covering the pigment is replaced by the resin solution. The solid/gas interface (pigment/air) is transformed into a solid/liquid interface (pigment/resin solution).
Grinding stage: By high shear forces the pigment agglomerates are broken up into smaller units, preferable primary particles.
Stabilization: The pigment dispersion is stabilized by dispersing agents in order to prevent the formation of uncontrolled flocculates. The resultant suspension is stabilized due to the adsorption of binder species or molecules at the pigment surface.
Dispersing additives, which adsorb on the pigment surface, facilitate liquid/solid interfacial interactions and help to replace the air/solid interface by a liquid medium/solid interface.
The grinding process can be regarded as a de-flocculation process. In the absence of stabilizing agents, effects such as reduced color strength, decreased gloss, and altered rheology may occur.
1.1 Stabilizing of Pigment dispersion
The pigment dispersion what is achieved in the last step will be used later in the let down system where it should stay stable during storage and later during the application and film formation.
There are two principal mechanisms for the stabilization of pigmented dispersions described:
1.1.1 Electrostatic stabilization is only working in a water based application. When two particles having the same charges approaching each other will result in a repelling effect. The resulting Coulomb-repulsion of the charged particles allows the system to remain stable.
1.1.2 Steric stabilization suited for water and solvent based systems is when pigments are sterically stabilized (the surface of the solid particles are completely covered by polymers) making particle-to-particle contact impossible. Strong interactions between polymers and solvents (organic solvent or water) prevent the polymers from coming too closely into contact with one another (flocculation).
Steric stabilization relies on the adsorption of a layer of resin or polymer chains on the surface of the pigment.
One fundamental requirement of steric stabilization is that the chains are fully solvated by the medium. This is important because it means the chains will be free to extend into the medium. In systems where the chains are not so well solvated they will prefer to lie next to each other on the surface of the pigment, providing a very much smaller barrier to inter-particulate attraction what will result in much easier flocculation
Dispersants Families
The choice of the dispersing agents for the pigment stabilization is a key issue in the coating and ink industry. Formulators have to find the most suitable products for their formulation taking into account the final application of their coating, the coating system (water based, solvent based, etc.) and the other additives.
The role of the dispersing agents is to enhance the dispersion process and ensure a fine particle size in order to stabilize pigments in the resin solution. As explained earlier, an efficient dispersant has to perform the three main functions: pigment wetting, dispersing, and stabilizing. Dispersing agents generally differ for aqueous and solvent-based coatings.
In term of chemical structure one can divide dispersing agents into the two following classes:
Surfactants, also called low molecular weight dispersing agents
The main differences of those two types of dispersants being the molecular weight, the stabilization mechanism and the resulting let down stability.
In addition polymeric dispersing agents have multiple anchor groups where surfactant like dispersing agents more related to a polar head with a side chain for the compatibility.
1.2 Polymeric dispersants
Polymeric dispersants stabilize paints, coatings and ink systems via a steric stabilization mechanism.
They must have specific anchor groups capable of being strongly adsorbed into the particle surface and must contain polymeric chains that give steric stabilization in the required solvent or resin solution system.
Polymeric dispersants differentiate themselves from the other types of dispersing agents through considerably higher molecular weights. Because of its structural features, a polymeric dispersant is bound to numerous sites at the same time, forming durable adsorption layers upon many pigment particles. Optimal steric stabilization is achieved when the polymer chains are well solvated and properly stretched, therefore they must be highly compatible with the surrounding resin solution. If this compatibility is obstructed, the polymer chains collapse causing the steric hindrance and the resulting stabilization to be lost.
In order for additives to be effective, the adsorption into the pigment surface must be durable and permanent. The surface properties of the pigment particles are therefore crucial to the additive’s effectiveness:
With pigments possessing high surface polarities, such as inorganic pigments that are ionically constructed, the adsorption of any dispersing additive is relatively easy.
However, for pigments with nonpolar surfaces, such as organic pigments whose crystals are composed of nonpolar individual molecules, a proper adsorption is rather difficult to obtain with conventional additives. The wide range of anchor groups that polymeric dispersants provide make them very efficient to anchor on pigments with nonpolar surfaces.
In the traditional method of stabilizing pigments in water, the stabilizing charges used are often disturbed by impurities, such as other ions, or the presence of other pigments with different zeta-potentials. This leads to a destabilizing effect, caused by the reduction of the repulsive forces. Steric stabilization can avoid this issue, making polymeric dispersants very useful for dispersing all types of pigments, even the organic ones, that are very difficult to be deflocculated by traditional wetting and dispersing additives.
The nature of the polymeric chain is critical to the performance of polymeric dispersants. If the chains are not sufficiently solvated, then they will collapse on to the pigment surface allowing the particles to aggregate or flocculate. The need for compatibility with the medium extends throughout the final drying stages of any applied coating. If it ceases to be compatible, flocculation may occur leading to a decrease of surface properties such as losses in gloss and tinctorial strength, etc.
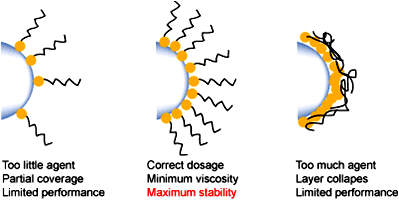
Finally, for good surface coating properties and performances, the polymer must be fully compatible with the coating resin after the solvent has evaporated off and the resin has been cross-linked.
1.3 surfactant dispersant
Surfactant dispersants are conventional low molecular weight dispersing agents. Surfactant molecules are able to modify the properties and, in particular, they lower the interfacial tension between the pigment and the resin solution.
This surface activity arises because the surfactants’ structure consists of two groups of contrasting solubility or polarity. In aqueous systems, the polar group is known as a hydrophilic group and the non-polar group as hydrophobic or lipophilic. In non-aqueous systems, the polar group is known as the oleophobic group and the non-polar group as oleophilic. Surfactants are classified according to their chemical structure and, more specifically, their polar group: anionic, cationic, electroneutral and non-ionic (see figure 1).
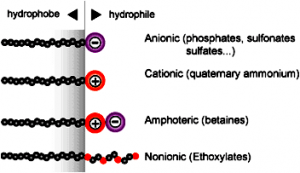
The absorption of the polar group onto the pigment surface. The anchoring groups can be amino, carboxylic, sulfonic, phosphoric acids or their salts.
• The behaviour of the nonpolar chain in the medium surrounding the particle. This part of the molecule (aliphatic or aliphatic-aromatic segments) must be highly compatible with the binder system.
The stabilisation mechanism of surfactant-like dispersing agents is electostatic: the polar groups forming an electrical double layer around the pigments particles. Due to the Brownian movement the pigment particles frequently encounter each other in the liquid medium thus having a strong tendency to re-flocculate on the let down stage.
Because of their chemical structure (eg: low molecular weigth) and the electrostatic method of stabilization, surfactants may cause the following defects:
• Water sensitivity: Surfactants generally have a tendency to provide water sensitivity to the final coating, thus making them inappropriate for use in outdoor applications.
• Foam formation: Many surfactants generate foams which lead to surface defects (eg. fish eyes, craters) on the final coating. If foaming occurs at the milling stage it can also cause a loss of production capacity.
• Interference with intercoat adhesion.
Over the past years specific surfactants have been developed to minimize these defects, and
some provide other advantages to the final paints such as defoaming/dearation or difficult substrate wetting.
The most widely used surfactants for pigment dispersion in coating formulations are:
• Fatty acid derivatives
• Phosphate esters
• Sodium polyacrylates / polyacrylic acid
• Acetylene diols
• Soya lecithin
2 Required amount of dispersant.
Dispersing agents are not just additives to conventional millbases. The choice of the most suitable dispersing agents is sometimes difficult and their usage require sometimes specific guidelines.
The choice of dispersant is also related to the surface nature of the pigment. The polarity of the surface of the pigment differs from organic (non-polar) to inorganic (more polar), and this means that the nature of the dispersant anchor group is critical for optimum absorbtion. The choice of anionic anchor group should allow for better performance with inorganic pigments and a cationic anchor group should be more appropriate for organic pigments.
The surface area of the pigment also affects the level of dispersant used, and in general, if too little is used then the full benefits will not be realised. If too much is used, it can be shown that the thickness of the protective barrier is actually reduced as a result of overcrowding on the pigment surface. Therefore the use of an excess level of dispersant actually leads to final coating properties which are inferior to those obtained with an optimised dosage.
As a general rule, 2-2.5mg of polymeric dispersant, per square meter of pigment surface area will be close to the optimum amount required.
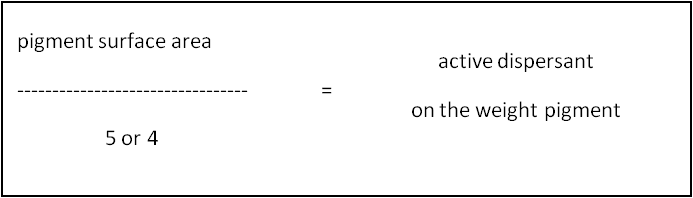
For example, the surface area of phtalocyanine blue pigment is 50 m2/g:
So a typical dosage would be :
• Phtalocyanine blue pigment 30.0 (i.e: 10% active dispersant on the weight of pigment)
• Polymeric dispersing agents: 3.0[:]